The Useful Water Reserve (RU) of a cultivated soil.
After intensive watering, the water occupies all the small cavities between the soil elements. After re-watering, which allows dry working, there is still water in the microspores smaller than 30 microns, while the macrospores are filled with air. The retention point is the amount of water that a soil can store when it is re-swept. If there is no further watering, the soil continues to lose water.
Beyond a certain dryness limit, plants are no longer able to extract water and start to wilt.
The useful reserve (or readily usable reserve) is the reserve between the point of water retention and the point where permanent wilting occurs. The water holding capacity varies according to the soil structure. The finer the texture of the soil, the higher the useful reserve (the more water the soil can hold). It is 80L/m3 for a sandy soil and 300L/m3 for a clay soil. A low retention capacity means that soluble fertilisers need to be applied in smaller doses to prevent them from being washed away. The knowledge of the useful reserve also makes it possible to foresee when it is necessary to proceed with watering and the periods of watering.
For more information, click here
Organic carbon content of a cultivated soil.
The determination of soil organic carbon is used to assess the total organic matter content of the soil. There are several methods of analysis by combustion or humic pathway to carry out the carbon determination. It is estimated that the organic matter/carbon ratio is approximately constant. Carbon is only a fraction of the organic matter. In laboratory analysis, a multiplication factor is applied to account for elements in the organic matter other than carbon, notably oxygen and nitrogen.
The organic matter content is obtained by multiplying the organic carbon content by a corrective factor generally between 1.7 and 2 depending on the laboratory. The coefficient 1.7 is the most commonly used. The organic carbon content (Corg or C in some lab analyses) is expressed as % of analysed soil. Other laboratories apply different corrective factors to go from the quantity of organic carbon to the organic matter content depending on the nature of the soil. for a soil containing humus of the morus type, the correction factor may be 1.77 to 1.93.
Measurement of phosphorus reserves in a cultivated soil.
Different determination methods are used depending on the soil pH. The Joret-Heber method consists of an extraction of assimilable phosphorus from ammonium oxalate in a neutral medium. The Joret-Heber method is considered satisfactory although it gives a lower result (about 1/3) than the more aggressive Dyer method. With the Joret-Heber method, the optimum is between 151 and 280, whereas in the Dyer method the optimum is estimated to be between 280 and 400. The Olsen method uses a reagent (sodium bicarbonate) which gives lower values than the two methods mentioned above.
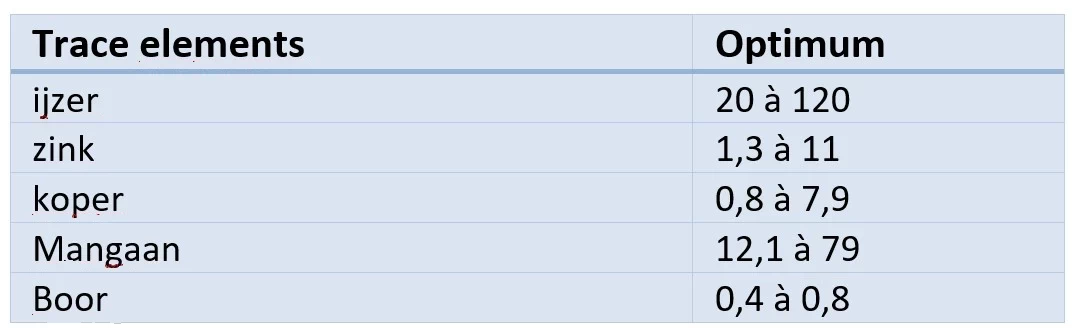
Fertilisation; measurement of some trace elements.
Trace elements are measured in mg per kg of dry matter according to the DTPA extraction method (AFNOR standard NF X 31-121). If the measurements show a trace element deficiency, this is easily corrected by specific foliar fertilisers acting directly on the plants (1). Books and websites may contain descriptions of micronutrient deficiencies using simple visual observations, which is not the best method because of the risk of confusion between real and induced deficiencies. This is even more true when there are several deficiencies. Only laboratory analysis can show with good precision the existence and origin of one or more deficiencies.
1) Foliar fertilization is not very effective in dry weather.
For more information on trace elements and fertilisation, click here
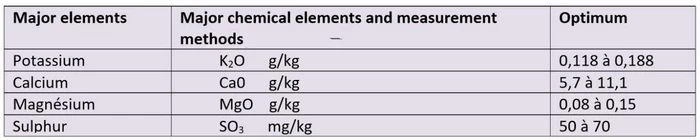
Measurement of some major elements of a cultivated soil.
The various major elements are evaluated in g per kg of soil or in mg per kg of soil
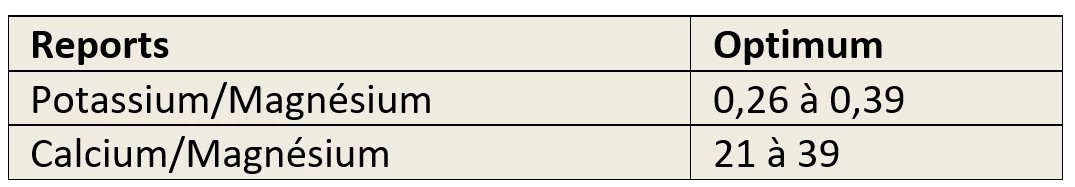
Fertilisation and specific ratios of potassium, magnesium, calcium.
The measurements should at least specify two ratios:
- Between potassium and magnesium,
- Between calcium and magnesium,
This is to ensure that there is no risk of induced deficiency.
Growing soil, measurement of total and free limescale.
Measuring total limestone means determining the amount of limestone present in the soil including the less water-soluble fraction. Part of the limestone is easily attacked by carbonic acid in rainwater or by organic acids in the soil to form soluble calcium bicarbonate producing calcium ions (Ca²+). This fraction of limestone is called active or free limestone and also includes other molecules such as calcium nitrate or monocalcium phosphates.
When Ca²+ cations are too abundant, they tend to saturate the absorbent complexes. Laboratory analyses allow us to determine the quantity of limestone present in the soil solution and the quantity of calcium ions fixed by the absorbent complexes. However, active limestone is far from being harmful as long as it remains incorporated in the soil water, as it maintains the coagulation of the clay. It is only when it comes into contact with the roots to form an asphyxiating sleeve that reduces iron absorption that limestone becomes toxic. In arboriculture, the knowledge of active limestone is essential to judge the fruit-bearing capacity of a soil. Tolerance standards are 15% for cherry, 10% for apple, 6 to 8% for pear and less for peach which is the most sensitive fruit species. For vegetables, most of which are calcareous, a soil rich in limestone, provided that it is correctly supplied with humus, is not very worrying. However, the addition of organic matter to a very calcareous soil can have a perverse effect when this organic matter contains humic acids which will dissolve the limestone and increase its solubility. To avoid this problem, a soil with too much limestone should be amended with clay before receiving organic matter.
Another form of limestone, gypsum (CaSO⁴), promotes plant growth when its content in the soil is 2 to 25%. Above this, gypsum can cause considerable reductions in crop yields. In soils dominated by active limestone, all inputs that could increase it should be excluded when it is not suitable for crop plants.
Above 5% of total limestone, which results in a systematically basic soil, the natural reserves of calcium and their progressive release by dissolution make rapid liming unnecessary. The determination of active limestone allows the definition of the Chlorosis Potency Index (CPI) which is an estimation of the risk of iron deficiency. A soil poor in free iron containing active limestone can result in a lack of iron assimilation (iron chlorosis). Some plants such as strawberry plants, grapevines and certain fruit trees such as apple trees are susceptible to this type of deficiency. When the active lime content is below 4%, the risk of iron chlorosis is very low. An active limestone content of more than 6% induces a significant risk of iron chlorosis which becomes very important when the content exceeds 10%.
Depending on the type of approval granted, some laboratories may produce other analyses, particularly on trace elements (cadmium, chromium, copper, nickel, lead, zinc, mercury) or use more precise methods for taking and storing soil samples for the determination of mineral nitrogen on fresh soil. These analyses should be undertaken when it is assumed that a newly acquired crop soil contains pollutants, or when one simply wants to know the content of certain trace elements.