The pH (hydrogen potential) describes the degree of acidity or alkalinity of the soil solution, which depends on the concentration of hydrogen ions. Soils are considered as :
Moderately acidic when the pH is below 6, and very acidic when the pH is below 5.
Moderately basic (alkaline) when the pH is above 8 and very basic when the pH is above 9.
A soil with low limestone content tends to be acidic (pH < 7). A soil rich in limestone is rather basic (pH > 7).
The pH value of the soil has an important effect on the biomass. Significant changes in the pH-value can result in a decrease of the bacterial biomass, which can be divided by up to 30.
A soil is naturally prone to acidification when it is based on a granitic, schistose or sandy substrate. Acidification is also a natural process in temperate climates, which accelerates in the rainy season. Over time, crop soils become acidified as a result of ammonium nitrification caused by bacteria when synthetic nitrogen fertilisers (containing urea, ammonium nitrate, ammonium sulphate, etc.) or organic fertilisers (manures, composts) are added to the soil. It should be noted that potassium or sodium nitrate, dicalcium phosphate and calcium cyanamide have an alkalinising effect when used alone.
Acidification is also higher in winter. A very wet and unventilated environment favours the production of organic acids. An acidic soil more easily produces an accumulation of undecomposed organic matter that facilitates the emergence of plant diseases.
Basic or acidic solution; what is the difference?
Any solution comprising water and a dissolved body (solute) is called an aqueous solution. All salts dissolved in water are in an ionic form. Ionic aqueous solutions contain ions with a negative electrical charge (anions) and ions with a positive electrical charge (cations). A solution is neutral when it contains as many anions as cations. An excess of hydrogen cations H+ is responsible for the acidity of a solution. When there are more hydroxide anions OH-, the solution is basic (alkaline).
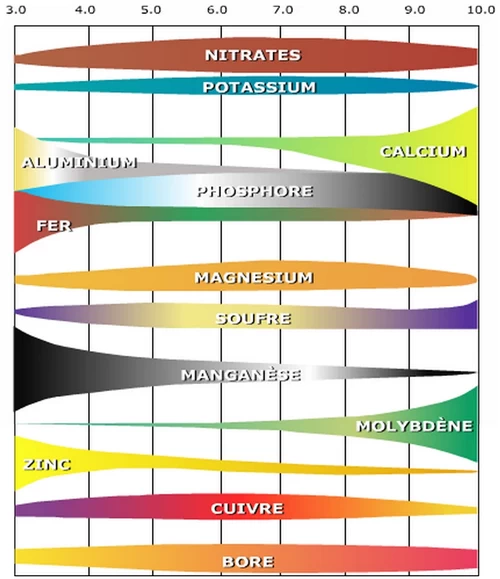
CThis diagram shows the impact of pH on the availability of nutrients and the emergence of toxicity of certain metals (notably aluminium) ♦
Source : Université Nice-Sophia Antipolis
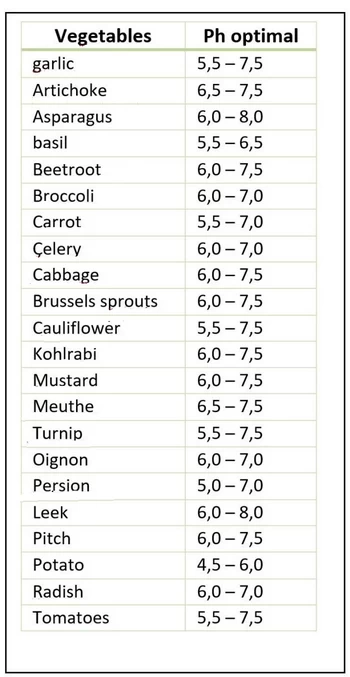
Optimal Absorption pH for some vegetables
pH and nutrient uptake in the soil.
The absorption of certain nutrients by plants depends on two factors linked to the soil pH:
The optimal soil pH for the absorption of each nutrient: Each nutrient has an optimal pH of availability for plants.
The optimum pH of nutrient uptake for each plant: The optimum pH for absorption varies according to the nature of the crop. For calcicultural crops, nutrients are best absorbed when the pH is above 8. For acidophilic crops, a pH below 6.5 is preferable.
In agronomy, fertility potential is considered unaffected when the pH is between 6.5 (slightly acidic) and 7.5 (slightly alkaline). Most nutrients are optimally absorbed by plants in this pH range, which is also compatible with their root growth. The activity of the microflora is more intense in a neutral environment. In the vicinity of neutrality (pH 7), microbial conversion of ammonia to nitrate is rapid. A neutral pH also favours the degradation of phytosanitary products.
A pH below 5 produces an excess of aluminium absorption which is toxic for plants; a risk evaluated at only 1% for French soils (1).
Plant uptake of nitrogen, potassium and sulphur seems to be less affected by soil pH. Phosphorus uptake, on the other hand, is more affected by the soil pH value. Most of the other nutrients (trace elements in particular) tend to be less available when the soil pH is above 7.5, whereas they are perfectly available when the pH is slightly acidic at 6.5 to 6.8. The exception is molybdenum (Mo), which is less available at acidic pH and more available at moderately alkaline pH values, which is very suitable for cucumber cultivation.
Soil pH plays an important role in ammonia volatilization losses. A nitrogen fertilizer such as urea is generally subject to greater losses at pH 8. However, there are other factors such as soil moisture, temperature, texture and cation exchange capacity that can affect ammonia volatilization. The important point to remember is that under conditions of low soil moisture or poor fertiliser incorporation, volatilisation loss can be considerable even if the pH falls as low as 5.5.
The soil pH is an important factor in nitrogen fixation in legumes. The survival and activity of the Rhizobium bacteria (responsible for nitrogen fixation in association with legumes) decreases with increasing soil acidity. This should be taken into consideration when trying to grow legumes (beans, peas...) on soils with a pH below 6.
In market gardening, a pH of 8 does not prevent acceptable harvests, even for potatoes, which normally prefer slightly acidic soils. In the Durance Valley, there are many farmers who produce different varieties of potatoes in limestone-rich soils with a pH of about 8. In general, these farmers have to deal with much more impactful problems such as pest pressure (in particular Colorado potato beetle and wireworm).
Taking into account all these elements mentioned above, a neutral value is a good average for all crops. This is the value that the amateur gardener should aim for by applying appropriate amendments.
Beware of the alkalinising effect of wood ash:
Wood ash, which contains a lot of potash and calcium, has an alkalinizing effect. Its action is very rapid and should be taken into account when spreading it on crops (or incorporating it into compost). In areas where the soil is rich in limestone and in the hope of improving its fertility, some amateur gardeners spread wood ash in their gardens when their soil is already alkaline. Of course, this addition, which increases the soil's pH, can have serious consequences on the soil's biodiversity and produce induced deficiencies of other elements. The amount of wood ash applied should not exceed 15 kg per 100 m².
Tip:
Wood ash contains potash which is very soluble. It is therefore not advisable to spread it in winter. As soon as it rains, the potash will be washed into the groundwater. It is best to add wood ash by scraping it into the top cm of the soil just before planting.
pH measurement for the home gardener
The vinegar test is sometimes mentioned on websites (2) to determine whether the soil is acid or alkaline. In reality, it mainly highlights the presence of limestone in the soil and does not give any precision on the pH value, nor on the free limestone, also called active limestone, present in the soil solution (which should not be confused with total limestone, part of which is in solid form). It is not necessary to go to a laboratory to find out the pH of the soil every time. There are electronic or chemical analysis tools available to the amateur gardener to measure the level of acidity or alkalinity of the soil.
Compatible electronic pH testers for agricultural activities
Adwa pH tester with calibration solutions
There are many models of pH testers at all prices (they are also used in aquariums, swimming pools, etc.). Most of these testers have to be calibrated with solutions. Some testers can be calibrated with a single solution (neutral pH) which is largely sufficient for agriculture. The acquisition cost for a first price tester is about 40 € with batteries and calibration solutions. For a slightly higher price, it is possible to acquire a model with a replaceable probe for example by clicking here where the probes are delivered calibrated. You can also order spare batteries and bags of calibration solution, which should be repeated every week. Do not buy a tester from a shop that does not supply calibration solutions. A translation of the instructions for the AD100 and 101 testers is available here.
Laboratory pH paper suitable for agricultural activities
There are pH paper strips impregnated with a reagent that colours differently depending on the pH value available here.